Chemistry Paper-2 2018 — CSS Past Paper
FEDERAL PUBLIC SERVICE COMMISSION
COMPETITIVE EXAMINATION-2018 FOR RECRUITMENT TO POSTS IN BS-17
UNDER THE FEDERAL GOVERNMENT
CHEMISTRY, Paper-2
TIME ALLOWED: THREE HOURS
PART-I(MCQS): MAXIMUM 30 MINUTES
PART-I (MCQS) MAXIMUM MARKS = 20
PART-II MAXIMUM MARKS = 80
NOTE:
- (i) Part-II is to be attempted on the separate Answer Book.
- (ii) Attempt ONLY FOUR questions from PART-II. ALL questions carry EQUAL marks.
- (iii) All the parts (if any) of each Question must be attempted at one place instead of at different places.
- (iv) Candidate must write Q. No. in the Answer Book in accordance with Q. No. in the Q.Paper.
- (v) No Page/Space be left blank between the answers. All the blank pages of Answer Book must be crossed.
- (vi) Extra attempt of any question or any part of the attempted question will not be considered.
PART-II
Q.No. 2.
- (a) Define Resonance and Resonance effect. (10)
- (b) Write Short note on followings. (5+5) (20)
- (i) Tautomerism
- (ii) Hyperconjugation.
Q.No. 3. (a) Complete the following reactions. (8×2=16)
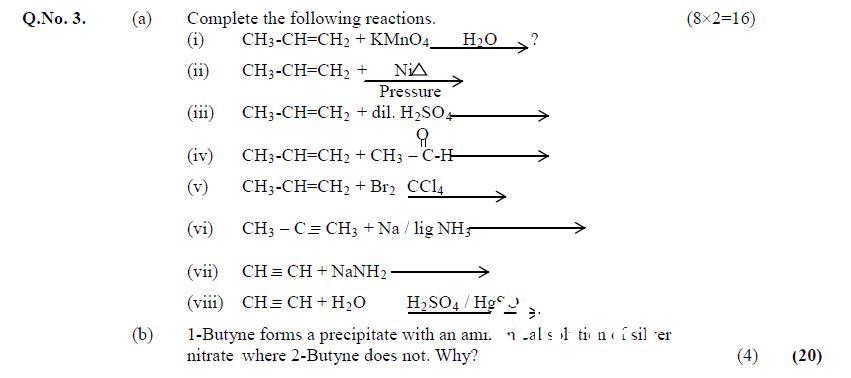
Question No 3, Chemistry Paper-2, CSS 2018
- (i) CH3-CH=CH> + KMnO4 H20 > ?
- (ii) CH3-CH=CH, + NiA Pressure
- (ii) CH3-CH=CH2 + dil. H2SO0,————_»> ?
- (iv) CH3-CH=CH, + CH3 — C-H—————->
- (v) CH3-CH=CH2 + Br2 CCly ;
- (vi) CH; —C= CH; + Na/lig N4;>-———>
- (vii) CH=CH+NaNH, ————>
- (viii) CH=CH+H20 H2804 /HgSO.,
(b) 1-Butyne forms a precipitate with an ammonical solution of silver nitrate where 2-Butyne does not. Why? (4) (20)
Q.No. 4. Explain electrophilic substitution reaction mechanism with the help of:
- (i) Nitration
- (ii) Sulphonation. (20)
Q.No. 5.
- (a) Distinguish between: (4×3=12)
- (i) Configuration and conformation
- (ii) Enantiomer and Diastreomers
- (iii) RR. Convention and S. Convention
- (b) Define specific rotation. How do you measure using polarimeter? (8) (20)
Q.No. 6.
- (a) What do you mean by the setting of cement. (10)
- (b) Discuss future of cement industry in Pakistan. (10) (20)
Q.No. 7.
- (a) Explain Aldol condensation reaction with examples. (10)
- (b) What are proteins? (5)
- (c) Explain Bio synthesis of cholesterol. (5)
Q.No. 8. Explain the following: (4 marks each) (20)
- (a) Beers Lamberts Law. (b) Wood Wards Fieser Rule
- (c) Hooks Law (d) Basic principle of NMR?
- (e) Chemical Shift.
**********
Most Popular
Tags
Botany
British History
Business Administration
Chemistry
Competitive Exam
Computer Science
Constitutional Law
Criminology
CSS
CSS-2021
CSS-2024
CSS 2022
Current Affairs
Economics
English
English Essay
English Literature
English Précis and composition
European History
FPSC
Gender Studies
General Science and Ability
Geography
Governance and Public Policies
History of Pakistan And India
International Law
International Relations
IR
Islamic History & Culture
ISLAMIC STUDIES
Islamiyat
Journalism & Mass Communication
Law
Mercantile Law
Pakistan Affairs
Pashto
Past Paper
Philosophy
Political Science
Psychology
Punjabi
Pure Mathematics
Sociology
Town Planning & Urban Management
Zoology