Chemistry Paper-2 2019 — CSS Past Paper
FEDERAL PUBLIC SERVICE COMMISSION
COMPETITIVE EXAMINATION-2019 FOR RECRUITMENT TO POSTS IN BS-17
UNDER THE FEDERAL GOVERNMENT
CHEMISTRY, Paper-2
TIME ALLOWED: THREE HOURS
PART-I(MCQS): MAXIMUM 30 MINUTES
PART-I (MCQS) MAXIMUM MARKS = 20
PART-II MAXIMUM MARKS = 80
NOTE:
- (i) Part-II is to be attempted on the separate Answer Book.
- (ii) Attempt ONLY FOUR questions from PART-II. ALL questions carry EQUAL marks.
- (iii) All the parts (if any) of each Question must be attempted at one place instead of at different places.
- (iv) Candidate must write Q. No. in the Answer Book in accordance with Q. No. in the Q.Paper.
- (v) No Page/Space be left blank between the answers. All the blank pages of Answer Book must be crossed.
- (vi) Extra attempt of any question or any part of the attempted question will not be considered.
PART-II
Q. No. 2.
- (a) Elaborate the optical isomerism with appropriate examples. (10)
- (b) Express the resolution and its applications. (5)
- (c) Explain the geometric isomerism in cyclic compounds. (5) (0)
Q. No. 3. (a) Prepare a plausible synthesis for each of the following transformation: (12)
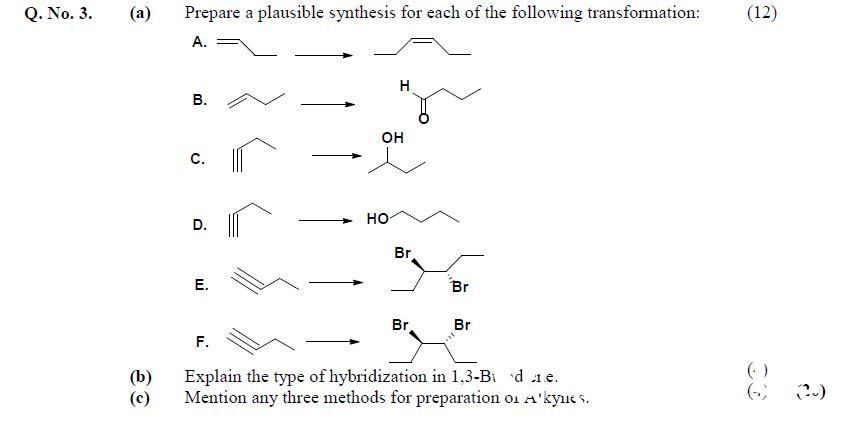
Question No 3, Chemistry Paper-2, CSS 2019
(b) — Explain the type of hybridization in 1,3-Butadiene. (4)
(c) Mention any three methods for preparation of Alkynes. (4) 0)
Q. No. 4.
- (a) Describe the necessary conditions and reagents required to convert benzene into the following.
Nitrobenzene, Ethyl — benzene, cyclohexane, Benz-aldehyde,
Benzoic acid, and Chlorobenzene. (8) - (b) Draw all possible structures of aromatic compounds withthe formula CoH, containing the benzene ring. (6)
- (c) | How do you account for the fact that phenol is more easily attacked by electrophiles than nitrobenzene? (6) (20)
Q. No. 5.
- (a) Outline stepwise reaction mechanism for the following reactions: (8)
- (i) SNI reaction between bromoethane and NaOH.
- (ii) SN2 reaction between 2-chloro-2-methyl propane and NaCN.
- (b) Discuss the various factors, nature of substrate, solvent, catalyst, and the leaving group in SN2 reaction.
- (c) How does methyl iodide react with the following reagents? (4) (20)
Acetic acid, Mg, Alcoholic KOH and Na.
Q. No. 6.
- Describe two methods for preparation of salicylic acid? How would you convert it into (a) Phenol, (b) Salol, (c) Benzoic acid and (d) Aspirin?
Give its at least two medicinal uses. - How will you obtain the following from suitable mono carboxylic acid?
(a) Iso-butane (b)Butanone (c)Benzamide (d) Propionaldehyde. - Describe the mechanism of esterification of an acid.
Q. No. 7.
- An unknown substance shows a molecular ion peak at m/z=170 witha
relative intensity of 100.The M+1 peak has relative intensity of 13.2 and
the M+2 peak has an intensity of 1.00. What is the molecular formula for
this substance? - Mention the various tools to interpret the mass spectra.
- What is the nitrogen rule? Explain it with suitable examples.
Q. No. 8.
- Elucidate the various steps involved in Glycolysis.
- Express the role of ATP in Glycolysis.
- Describe the pathway that leads to the formation of Lactic acid.
**********
Most Popular
Tags
Botany
British History
Business Administration
Chemistry
Competitive Exam
Computer Science
Constitutional Law
Criminology
CSS
CSS-2021
CSS-2024
CSS 2022
Current Affairs
Economics
English
English Essay
English Literature
English Précis and composition
European History
FPSC
Gender Studies
General Science and Ability
Geography
Governance and Public Policies
History of Pakistan And India
International Law
International Relations
IR
Islamic History & Culture
ISLAMIC STUDIES
Islamiyat
Journalism & Mass Communication
Law
Mercantile Law
Pakistan Affairs
Pashto
Past Paper
Philosophy
Political Science
Psychology
Punjabi
Pure Mathematics
Sociology
Town Planning & Urban Management
Zoology